Comprehensive Services for the Pharmaceutical Industry: Cloud Solutions, Validation and Quality Systems

Pharmaceutical Cloud
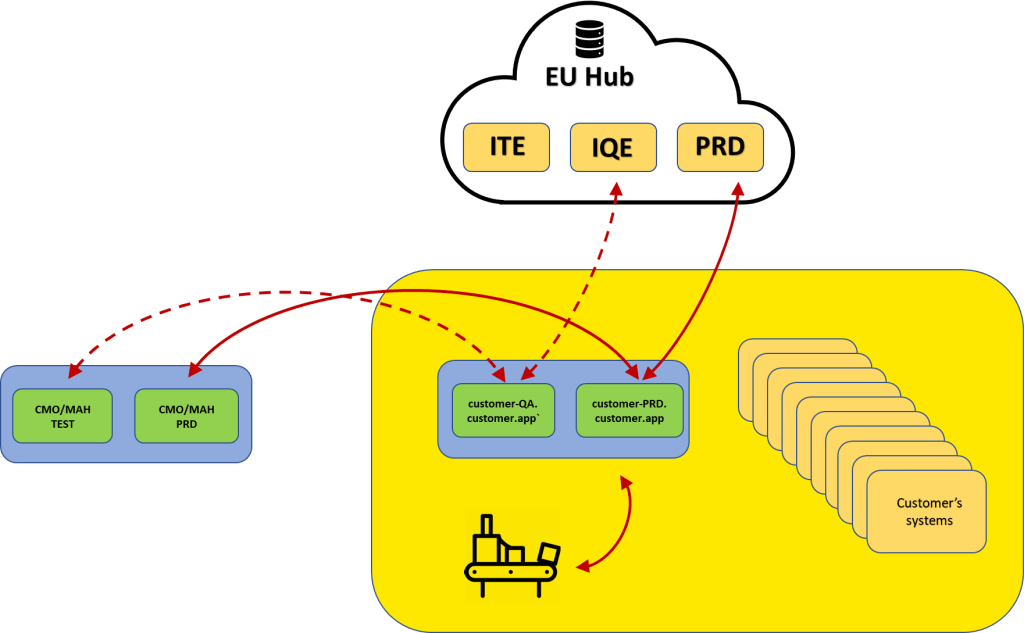
The Pharmaceutical Cloud Introl Cloud is a dedicated hardware space PaaS for computerised systems used in industry. The solution provides outsourcing of IT department tasks and qualifications for security and infrastructure maintenance. The security used and the way back-ups are taken and stored both ensure data security and meet all industry requirements. Additional components deployed in the cloud enable external system integrations with both central and national data repositories and contractual partners.
- The main advantages of the Pharmaceutical Cloud include:.
- compliance with legal requirements,
- certainty of data protection and security
- reduction of IT licences and hardware costs,
- outsourcing of IT services.

Validation
We have our own team carrying out qualification and validation work for ongoing projects and revalidation of existing systems. Expert staff have over 15 years’ experience working in the EU GMP environment.
In addition, as part of our qualification and validation work, we provide services such as:.
- cleaning validation,
- cross-contamination,
- quality risk management,
- preparing plants for inspections,
- resolution of reported problems – GAP analysis,
- process validation and optimisation of manufacturing processes.

Consulting
We verify, analyse processes, data to meet legal requirements and qualitative acceptance criteria. We verify, analyse and check design solutions for room concepts, HVAC installations and automation guidelines HVAC, RMS, BMS against legal requirements and qualitative customer acceptance criteria. We provide solutions to optimise processes in line with available technology. We perform a gap analysis. We provide solutions to optimise processes in line with available technology. We identify risks and ways to reduce them. We support process managers and process owners by presenting the status quo and the necessary optimal, responsible actions to improve compliance with EU GMP requirements.
…today we plan what tomorrow will look like
- objective process evaluation supports the soundness of the decisions of those in charge,
- we provide evaluation results and solution proposals,
- we help develop and implement optimal improvement and development plans.

Outsourcing
Providing Quality Assurance services – we provide support in process validation, preparation for inspections, periodic reviews, qualification/requalification of buildings, computerised systems, ancillary systems – HVAC, purified water, compressed air and others, metering systems, inspections, change control, CAPA, risk management including cross-contamination risk management, cleaning validation and others.
We provide meritorial support and assistance with stacking work developing documentation. We have experience of improvements and facilitation in maintaining the validated/qualified status of medicinal products and supporting systems. We optimise operations according to the requirements of EU GMP.
- Risk management
- Consulting, advisory and optimisation
- Validation/revalidation
- External audits and inspection support

Inspection and the pharmaceutical quality system
The pharmaceutical Quality System is the backbone of a pharmaceutical company. However, not only specialised knowledge and experience, but also objective assessment of the level of implementation and maintenance is required to improve it and plan directions.
- Determining areas of manufacturing requiring preparation for
Inspection according to customer needs. - Determination of the scope of legal requirements and standards supporting compliance
with the requirements (list of products manufactured , processes carried out,
special conditions, etc.). - Establishment of reference documentation providing evidence of compliance
with the requirements
(procedures, instructions plans , protocols , reports etc.). - Establish a secure way to share documentation.
- Develop a requirements matrix to record the results of the remote audit:
– data entry,
– quantification of meeting requirements in %,
– designation of areas for improvement. - Proposals for CAPA urgent actions and long-term development strategies.